Urea, a white crystalline solid containing 46% nitrogen, is widely used in the agricultural industry as an animal feed additive and fertilizer Here we discuss it only as a nitrogen fertilizer.
Urea (46-0-0) usually has the lowest cost per pound of nitrogen compared to other single-element nitrogen fertilizers. However, urea undergoes unique chemical transformations when field applied and severe losses in efficiency may result if special management practices are not followed. The purpose of this fact sheet is to briefly describe urea transformations and to suggest how urea-N may be conserved with proper management in the field.
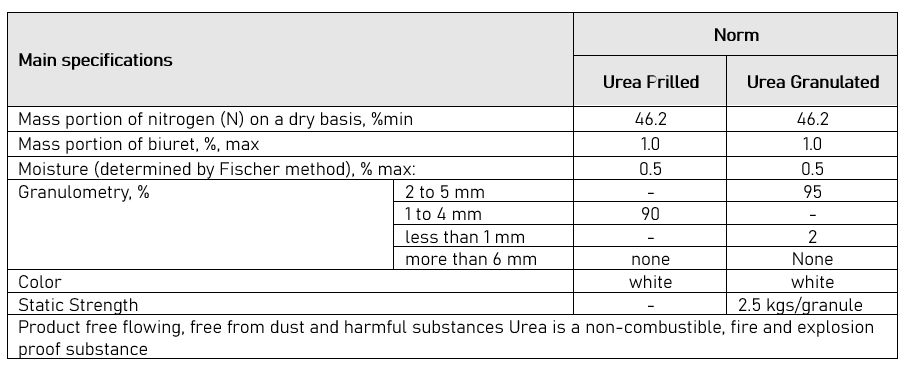
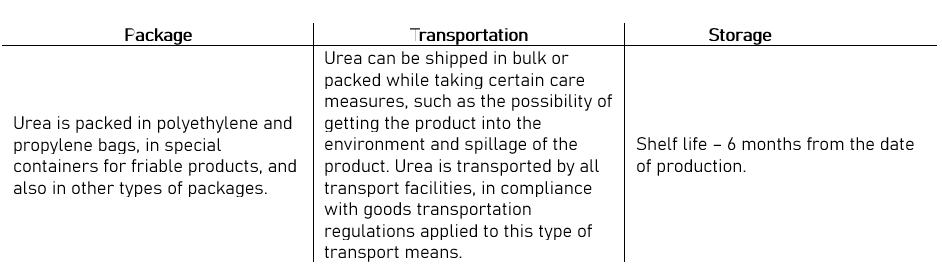
Fertilizer urea can be purchased as prills or as a granulated material. In the past, it was usually produced by dropping liquid urea from a “prilling tower” while drying the product. The prills formed a smaller and softer substance than other materials commonly used in fertilizer blends. Today, though, considerable urea is manufactured as granules. Granules are larger, harder, and more resistant to moisture. As a result, granulated urea has become a more suitable material for fertilizer blends.
Product Description
In common with most commercial nitrogen fertilizers, urea is manufactured from anhydrous ammonia (NH3). The high analysis of urea—46% N—is the main reason for the low cost of this form of nitrogen fertilizer. Freight costs and storage and handling are all lower than with lower analysis fertilizers such as ammonium nitrate (34-0-0) or ammonium sulfate (21-0-0).
Advantages of Fertilizer Urea
- can be applied to soil as a solid or solution or to certain crops as a foliar spray.
- Urea usage involves little or no fire or explosion hazard.
- Urea’s high analysis, 46% N, helps reduce handling, storage and transportation costs over other dry N forms.
- Urea manufacture releases few pollutants to the environment.
- Urea, when properly applied, results in crop yield increases equal to other forms of nitrogen.
- Incorporate urea for best use
Nitrogen from urea can be lost to the atmosphere if fertilizer urea remains on the soil surface for extended periods of time during warm weather. The key to the most efficient use of urea is to incorporate it into the soil during a tillage operation. It may also be blended into the soil with irrigation water. A rainfall of as little as 0.25 inches is sufficient to blend urea into the soil to a depth at which ammonia losses will not occur.
If properly applied, urea and fertilizers containing urea are excellent sources of nitrogen for crop production. After application to the soil, urea undergoes chemical changes and ammonium (NH4 +) ions form. Soil moisture determines how rapidly this conversion takes place.
When a urea particle dissolves, the area around it becomes a zone of high pH and ammonia concentration. This zone can be quite toxic for a few hours. Seed and seedling roots within this zone can be killed by the free ammonia that has formed. Fortunately, this toxic zone becomes neutralized in most soils as the ammonia converts to ammonium. Usually it’s just a few days before plants can effectively use the nitrogen. Although urea imparts an alkaline reaction when first applied to the soil, the net effect is to produce an acid reaction.
Urea or materials containing urea should, in general, be broadcast and immediately incorporated into the soil. Urea-based fertilizer applied in a band should be separated from the seed by at least two inches of soil. Under no circumstances should urea or urea-based fertilizer be seed-placed with corn.
With small grains, 10 lb. of nitrogen as urea can generally be applied with the grain drill at seeding time even under dry conditions.
